Title: Distinguishing Genuine Vaccines from Counterfeits Using MALDI-MS Technology
Introduction and Background
A recent study utilized Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS) to analyze four authentic vaccines alongside eight surrogates known to resemble falsified products. The genuine vaccines included Nimenrix (Pfizer Ltd) for meningitis, Engerix B (GlaxoSmithKline) for hepatitis B, Flucelvax Tetra (Seqirus Ltd) for influenza, and Ixiaro (Valneva Ltd) for Japanese encephalitis. The findings emphasize the detection capabilities of MALDI-MS in distinguishing between authentic vaccines and their falsified counterparts through a detailed analysis of their mass spectral profiles.
Methodology
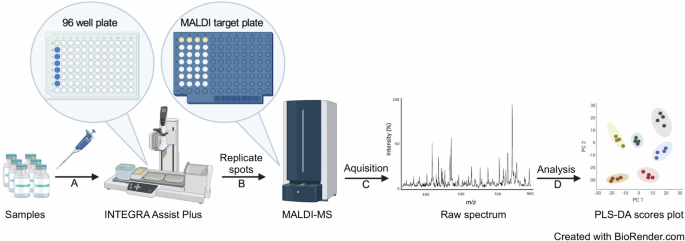
Two MALDI-MS instruments, the Biotyper Sirius (Bruker Daltonics) and the VITEK MS (bioMérieux), were employed for parallel analysis of samples, highlighting their comparable performance. Modifications to laser settings allowed for the capture of mass spectra across m/z ranges of 0–900, 700–2500, and 2000–20,000, with an initial focus on the 0–900 range to identify specific vaccine excipients. The researchers used manual inspection to differentiate unique mass spectral peaks present in genuine vaccines and known falsified samples.
Reproducibility and Validation
To assess reproducibility, the researchers evaluated both "spot-to-spot" and intra-batch variability by creating multiple replicate spots for each vaccine and surrogate. A pooled quality control sample was also prepared to ensure consistency across analyses. Results indicated that most samples demonstrated relative standard deviations (RSD) between 18% and 44%. Notably, one sample, Amikacin, exhibited an abnormally high RSD, likely due to poor sample crystallization affecting ionization efficiency.
Data Processing and Statistical Analysis
The study further developed a data processing workflow using the MALDIquant R package to enhance the accuracy of spectral analysis. This included baseline correction and peak normalization to address variances in intensity observed across samples. Using hierarchical clustering and Partial Least Squares Discriminant Analysis (PLS-DA), the team successfully distinguished authentic vaccines from falsified constituents by identifying significant mass spectral peaks.
Machine Learning Integration
The research employed machine learning techniques to create a robust classification model distinguishing authentic vaccines from fakes. This model underwent rigorous validation, demonstrating accuracy and reliability in predicting authenticity across all tested samples. The top 15 discriminatory peaks, identified through Variable Importance in the Projection (VIP) plots, could significantly differentiate genuine vaccines from their counterparts.
Conclusion and Implications for Public Health
The findings signify a promising advancement in vaccine authentication, showcasing how MALDI-MS can be implemented in screening for counterfeit vaccines in supply chains. The research indicates potential for establishing a comprehensive database of mass spectral peaks to ensure vaccine integrity, crucial for maintaining public trust and safety in immunization programs.
Overall, this study not only underscores the efficacy of MALDI-MS in forensic applications within the pharmaceutical industry, but also establishes a methodological framework that could serve as a benchmark for future vaccine authentication efforts globally.